Magnesium sulfide is colorless to brown crystal powder, which is sulfide of magnesium. It is of sodium chloride type structure and the chemical property is similar to other ionic type sulfide compounds as sodium sulfide, barium sulfide and calcium sulfide. Magnesium metal and pure sulfur are the raw materials. It dissolve in diluted hydrochloric acid, however MgS crystal react with water slowly.
Magnesium Sulphide (MgS) is an inorganic compound with unique optical and thermal properties, making it valuable across several advanced technology sectors. With a wide bandgap and high stability, magnesium sulphide serves an important role in optoelectronic devices, high-performance ceramics, and applications that require materials capable of withstanding extreme temperatures.

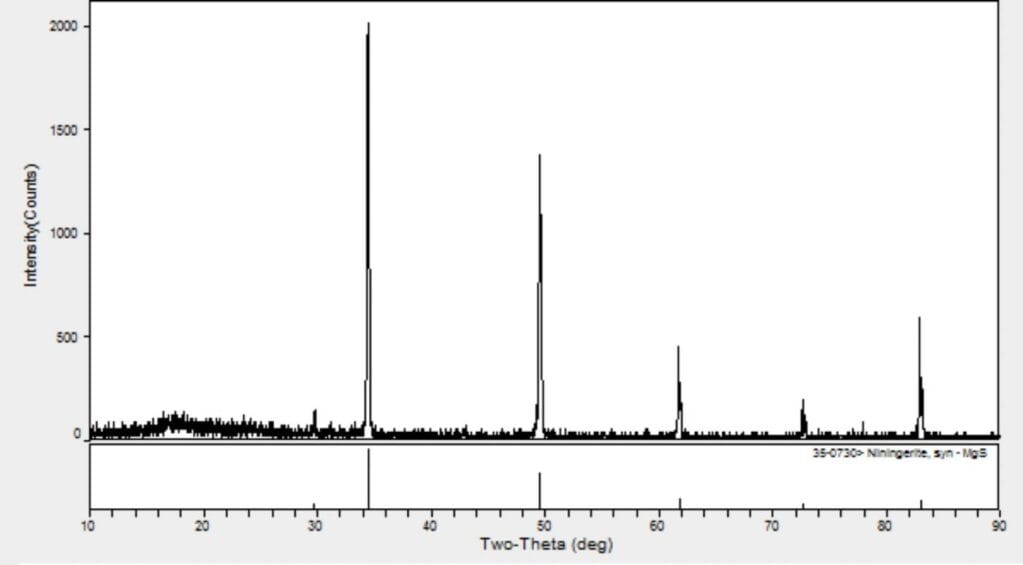

Magnesium Sulfide
| CAS No.:12032-36-9 | EINECS No.:234-771-1 | Molecular Formula:MgS | Molecular Weight:5638 |
| Melting Point:2000℃ | Density:2.68 |
Applications
Magnesium sulphide can apply to do desulfurization for iron and steel and making fluorescent powder. It can apply as a semiconductor with wide band gap.
In optoelectronics, magnesium sulphide is used as a phosphor material due to its luminescent properties. MgS can emit light when excited. It makes it suitable for devices like LEDs and display technologies that rely on efficient light emission. Additionally, magnesium sulphide get use in infrared (IR) sensors and detectors. It benefits applications in night vision, thermal imaging, and environmental monitoring, where infrared transparency and stability are crucial.
In ceramics, MgS is valueable for its ability to enhance the performance of high-strength, high-temperature ceramics. These ceramics are essential in industries such as aerospace and automotive manufacturing, where materials must maintain strength and stability under harsh conditions. MgS can be incorporated into ceramic composites to improve their thermal resistance and mechanical properties. It extends their durability and performance in challenging environments.
